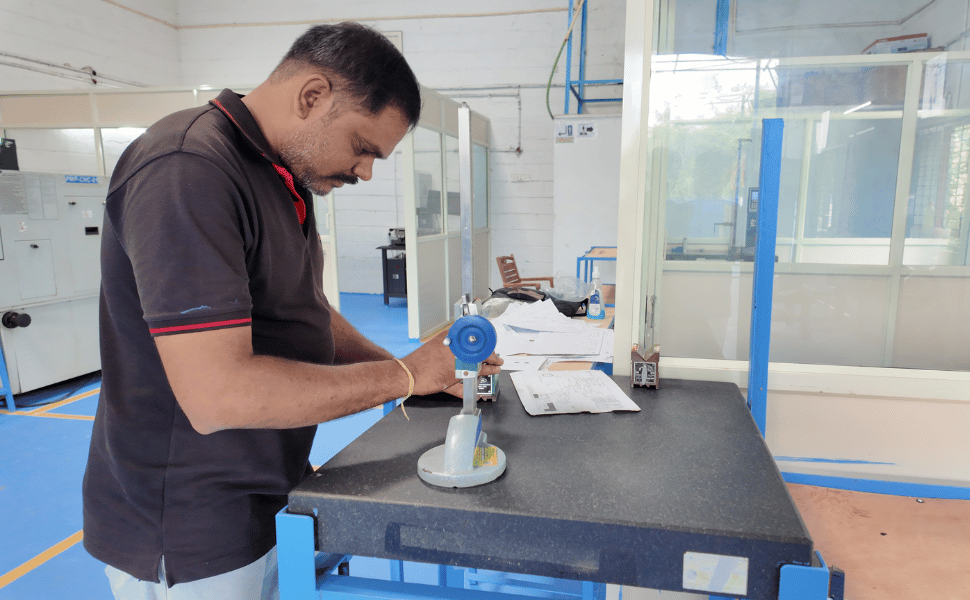
Quality Control
At PMP Precision Engineering, quality is not just a checkpoint — it’s a culture, a mindset, and a non-negotiable promise. In industries such as aerospace, defense, medical, and automotive, we understand that even the smallest deviation can lead to significant consequences. That’s why every component we manufacture undergoes rigorous quality control protocols to ensure complete compliance with client specifications and global standards.
Our quality assurance process begins well before the first cut is made. From raw material verification and detailed process planning to in-process inspections and final audits, every stage is meticulously monitored and documented. We utilize advanced inspection equipment, including CMMs, surface finish testers, and high-precision gauges, to validate critical dimensions and tolerances.
Backed by ISO-certified systems, our experienced quality team works closely with production and engineering departments to ensure flawless execution at every step. This integrated, end-to-end approach allows us to consistently deliver zero-defect components, enhancing the reliability, safety, and performance of every product we produce.
At PMP Precision Engineering, quality is engineered into every part — because our clients deserve nothing less than perfection.
Inspection Equipment
Our Quality Control Lab is Equipped With
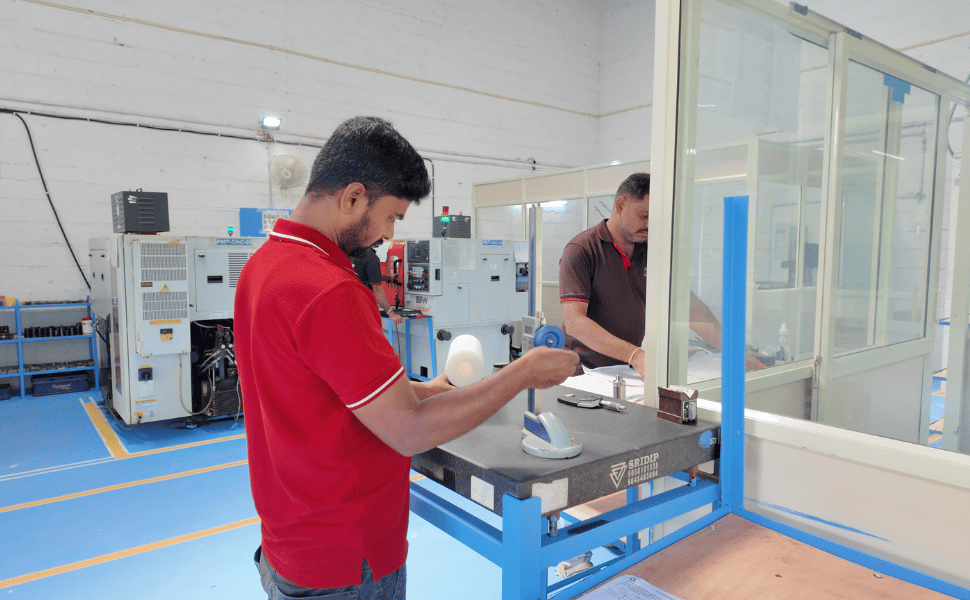
Our Commitment
Our Quality Standards
Our Quality Assurance Pillars
At PMP Precision Engineering, our quality assurance framework is built on four key pillars that ensure every component meets the highest standards of precision, consistency, and compliance.
Dimensional Accuracy – We utilize calibrated measuring tools, gauges, and advanced inspection systems to validate every dimension against design specifications, ensuring exacting tolerances are achieved in every part.
Process Control – Quality is integrated into every stage of production. From raw material verification to in-process checks and final inspections, each step is governed by strict quality checkpoints and protocols.
Documentation & Traceability – Every component is uniquely tagged and documented, ensuring complete traceability throughout its lifecycle. This enables accurate audits, non-conformance analysis, and full regulatory compliance.
Certifications & Standards Compliance – Our quality management systems align with ISO and industry-specific standards, ensuring globally compatible, reliable, and audit-ready processes and products.